Observations-
Tubes with different gases make different colors
1. Neon-
when i put on the glasses i see diferent colors all over the room. I can see rainbow colors coming off my computer screen. I see red, yellow, and orange lines coming off of the neon light at the top, bottom, left and right, and the corners of the light
2. Helium-
I see rainbow colors all over the room, but i do not the red, yellow, and orange colors to the right, left, top and bottom of the light. The colors coming off the light are blue, green, and yellow.. and maybe red at the very tips of it.
3. Argon-
I see purple and rainbow color lines coming off the top, bottom, left and right sides of the light. This one was very hard to see.
4. Nitrogen-
I see the colors coming off of this one really well, I see lots of colors coming of the the top, bottom, left, and right.
5. Carbon Dioxide-
This one looks just like the last one. I can see the colors in the same place and they look like the same colors. The one before this one looked brighter than this one.
Thursday, January 20, 2011
Activity 2 1-20-11 Antacids
Write the formula for Tums? How does Tums chemically react with water and stomach acid?
Limestone
Chalk
Insoluble compound in H2O "Sizzling"
CO3-2 + H+ -------> HCO3- + H+ -------> H2CO3 --------------> H20 + CO2 Evolution of Gas
Carbonate Bicarbonate Carbonic Acid <-------------- Carbon Dioxide Gas
CaCO3----------> Ca(aq)+2 + CO3(aq)-2
CalciteLimestone
Chalk
Insoluble compound in H2O "Sizzling"
CO3-2 + H+ -------> HCO3- + H+ -------> H2CO3 --------------> H20 + CO2 Evolution of Gas
Carbonate Bicarbonate Carbonic Acid <-------------- Carbon Dioxide Gas
Tums contain calcium cabronate. Calcium carbonate works by binding to excess acid produced by the stomach. This neutralises the acid and decreases the acidity of the stomach contentsAntacids are medications that increase the pH balance in your stomach. A number of symptoms, including heartburn, gastritis, and gastroesophageal reflux disease (GERD), can be treated with them. In most cases, antacids start working within a few minutes. It is important to note that they may not always be necessary, and they can have serious consequences if used improperly.
How many Tums are needed to neutralize a can of coke?
Activity 1 1-20-11 Experimental Design and Conclusions
Reflect on the expansion of water and salt water experiments you and your classmates performed. What are some of the interesting results and struggles with this experiment.
I think there is so much controversy about the green house effect and "is the earth warming" because everyone's experiments on the green house effect is different. Today in class we talked about adding salt to water and what happens when it freezes and everyones experiment was different. The data collected on the salt water experiment was different. That being said, I think researchers are finding different results and to some people the green house effect is a really big deal and to others its not a big deal at all. All of the experiements on "is the earth warming" are all different and that makes it hard to come to a conclusion about it.
The water/salt water experiements had interesting results. Before doing this experiment I didnt know that salt would make water take longer to freeze. It was hard to measure the results for expanison. I could see that my cups expanded but I didnt know how to measure it. Another thing I noticed about the experiements was everyone in the class had different results. Also everyone in the class did their experiment alittle different. I would have liked to work with other people on this experiment and see what 2 minds put together would have come up with. We learned today that there are lots of ways to do experiments.
Relate your thoughts on this experiment to the scientific question "Is the earth warming?"
I think there is so much controversy about the green house effect and "is the earth warming" because everyone's experiments on the green house effect is different. Today in class we talked about adding salt to water and what happens when it freezes and everyones experiment was different. The data collected on the salt water experiment was different. That being said, I think researchers are finding different results and to some people the green house effect is a really big deal and to others its not a big deal at all. All of the experiements on "is the earth warming" are all different and that makes it hard to come to a conclusion about it.
Wednesday, January 19, 2011
Homework 2 1-19-11 Stuff around us? and last homework
For your last homework assignment, choose 20 items around you in your world and list their ingredients or materials down to the molecular level. List the chemical names of the ingredients and show a molecular structure image.
1. Apple Juice
Filtered water, apple juice concentrate, and ascorbic acid (vitamin c)
C6H8O6

2. 7 Up
Filtered carbonated water, high fructose corn syrup and less than 2 % of natural flavors, citric acid, potassium citrate, calcium disodium edta

3. Salt
Salt, calcium silicate, dextrose potassium iodide
NaCl
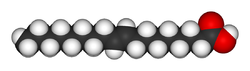
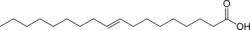
4. Balsamic Vinegar
Grape must, wine vinegar, caramel color, contains naturally occurring sulfites
C2H4O2

5. Baking Powder
Cornstarch, bicarbonate of soda, sodium aluminum sulfate, acid phosphate of calcium
NaHCO3
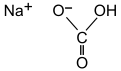
6. All Vegetable Shortening
Partially hydrogenated soybean oil and partially hydrogenated cottonseed oil, mono-and diglycerides added
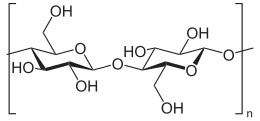
7. Corn Starch
Corn starch
(C6H10O5)n

8. Baking Soda
Sodium Bicarbonate
H2CO3 +2 H2O ↔ HCO3− + H3O+ + H2O ↔ CO32− +2 H3O+
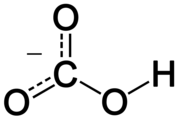
9. Celery Salt
Salt and celery seed
C2O42−
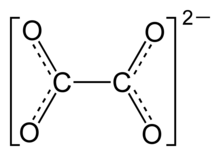
10. Seasoned Salt
Salt, sugar, spiced (including paprika, turmeric) onion, cornstarch, garlic, tricalcium phosphate, natural flavor
2HSCH2– CHNH2– COOH + R–S(O)S–R' → R–S–S–CH2CHNH2COOH + R'–S–S–CH2CHNH2COOH

11. 1% Lowfat Milk
Lowfat milk, vitamin A palmitate, vitamin D3
12. Mayonnaise
Soybean oil, water, eggs, distilled vinegar, salt, sugar, lemon juice, oleoresin paprika, natural flavors
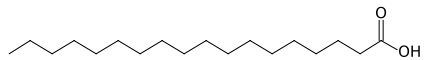
13. Dill pickles
Cucumbers, water, distilled vinegar, salt, calcium chloride, polysorbate 80, natural spice flavors, tumeric oleoresin

14. Lemon-Lime Gatorade
Water, sucrose, dextrose, citric acid, natural flavor, salt, sodium citratem monopotassium phosphate, gum arabic, glycerol ester of rosin, yellow 5

15. Kraft Singles
Cheddar cheese, milk, whey, milkfat, milk protein concentrate, salt, calcium phosphate, sodium citrate, whey protein concentrate, sodium phosphate, sorbic acid as a preservative, apocarotenal (color), annatto (color), enzymes, vitamin D3, cheese culture, yellow dye.

16. Welch's Fiber 100% Grape Juice
1. Apple Juice
Filtered water, apple juice concentrate, and ascorbic acid (vitamin c)
C6H8O6

2. 7 Up
Filtered carbonated water, high fructose corn syrup and less than 2 % of natural flavors, citric acid, potassium citrate, calcium disodium edta
C8H3Mg2
C6H5K3O7

3. Salt
Salt, calcium silicate, dextrose potassium iodide
NaCl

4. Balsamic Vinegar
Grape must, wine vinegar, caramel color, contains naturally occurring sulfites
C2H4O2

5. Baking Powder
Cornstarch, bicarbonate of soda, sodium aluminum sulfate, acid phosphate of calcium
NaHCO3

6. All Vegetable Shortening
Partially hydrogenated soybean oil and partially hydrogenated cottonseed oil, mono-and diglycerides added
7. Corn Starch
Corn starch
(C6H10O5)n

8. Baking Soda
Sodium Bicarbonate
H2CO3 +2 H2O ↔ HCO3− + H3O+ + H2O ↔ CO32− +2 H3O+

9. Celery Salt
Salt and celery seed
C2O42−

10. Seasoned Salt
Salt, sugar, spiced (including paprika, turmeric) onion, cornstarch, garlic, tricalcium phosphate, natural flavor
2HSCH2– CHNH2– COOH + R–S(O)S–R' → R–S–S–CH2CHNH2COOH + R'–S–S–CH2CHNH2COOH

11. 1% Lowfat Milk
Lowfat milk, vitamin A palmitate, vitamin D3

12. Mayonnaise
Soybean oil, water, eggs, distilled vinegar, salt, sugar, lemon juice, oleoresin paprika, natural flavors

13. Dill pickles
Cucumbers, water, distilled vinegar, salt, calcium chloride, polysorbate 80, natural spice flavors, tumeric oleoresin

14. Lemon-Lime Gatorade
Water, sucrose, dextrose, citric acid, natural flavor, salt, sodium citratem monopotassium phosphate, gum arabic, glycerol ester of rosin, yellow 5

15. Kraft Singles
Cheddar cheese, milk, whey, milkfat, milk protein concentrate, salt, calcium phosphate, sodium citrate, whey protein concentrate, sodium phosphate, sorbic acid as a preservative, apocarotenal (color), annatto (color), enzymes, vitamin D3, cheese culture, yellow dye.
16. Welch's Fiber 100% Grape Juice
Grape Juice from Concentrate (Filtered Water, Grape Juice Concentrate)Grape Juice, Maltodextrin (Dietary Fiber)Ascorbic Acid (Vitamin C)

17. Ketchup
Tomato concentrate made from red ripe tomatoes, distilled vinegar, high fructose corn syrup, corn syrup, salt, spice, onion powder, and natural flavors

18. Smucker's Concord Grape Jelly
Concord grape juice, high fructose corn syrup, fruit pectin, citric acid, sodium citrate

19. Peanut Butter
Peanuts, dextrose, hydrogenated vegtable oil (cotton seed and/or rapeseed) and salt

17. Ketchup
Tomato concentrate made from red ripe tomatoes, distilled vinegar, high fructose corn syrup, corn syrup, salt, spice, onion powder, and natural flavors
18. Smucker's Concord Grape Jelly
Concord grape juice, high fructose corn syrup, fruit pectin, citric acid, sodium citrate
19. Peanut Butter
Peanuts, dextrose, hydrogenated vegtable oil (cotton seed and/or rapeseed) and salt
20. Hidden Valley Ranch Dressing
Vegetable Oil (Canola and/or Soybean Oil) Water, Egg Yolk, Sugar, less than 2% of Buttermilk, Salt, Lactic Acid, Vinegar, Modified Food Starch, Disodium Inosinate and Disodium Guanylate, Dried Garlic, Dried Onion, Phosphoric Acid, Monosodium Glutamate, Xanthan Gum, Natural and Artificial Flavors, Spices, Disodium Phosphate, Sorbic Acid and Calcium Disodium Edta As Preservatives.
Homework 1 1-19-11
Use the pH paper to develop a table and graph of the pH of common things around your home. Think in terms of at least 10 different liquids.
1. Milk pH 6
2. Lemon lime gatorade pH 4
3. Pickle juice pH 5
4. Diet cranberry juice pH 3
5. Wild island boones farm pH 5
6. Dawn dish soap pH 8
7. Balsamic vinegar pH 4
8. Cidar vinegar pH 3
9. White distilled vinegar pH 5
10. 7 up pH 4
Develop an experiment to see how much antacid is needed to neutralize an acidic liquid.
I place 1/4 cup of diet cranberry juice in a cup and added 1 crushed up tums. I stirred the cranberry juice with the tums in it and then waited 5 minutes before checking the pH. The pH of the cranberry juice was 3 but after added the tums the pH was a 7. The tums worked!
2. Lemon lime gatorade pH 4
3. Pickle juice pH 5
4. Diet cranberry juice pH 3
5. Wild island boones farm pH 5
6. Dawn dish soap pH 8
7. Balsamic vinegar pH 4
8. Cidar vinegar pH 3
9. White distilled vinegar pH 5
10. 7 up pH 4
Develop an experiment to see how much antacid is needed to neutralize an acidic liquid.
I place 1/4 cup of diet cranberry juice in a cup and added 1 crushed up tums. I stirred the cranberry juice with the tums in it and then waited 5 minutes before checking the pH. The pH of the cranberry juice was 3 but after added the tums the pH was a 7. The tums worked!
Homework 1-18-11
When is water the smallest?
Water is smallest when it is at liquid form at room temperature
What happens when salt is added to the water?
When salt is added to the water it takes a lot longer for the water to completely freeze.
Does the amount of salt added affect the freezing?
Yes, the more salt in the water the longer it take for the water to freeze.
For the experiment I had 3 plastic cups with 1 cup of water in each cup. I let the water sit at room temperature for 1 hour. The water's temperature was 16C. I marked the cups at the water level.
Cup 1- Water
Cup 2- Water with 1 shake of salt
Cup 3- Water with 1 spoonful of salt
I placed all 3 cups into the freezer. The freezers temp was -15 C. I left the cups in the freezer for 7 hours. When I checked the cups the water was frozen in all the cups except for cup 3, that was the cup with a spoonful of salt. Cup 3 had a small layer of non-melted water sitting on top of the ice. I noticed that the plastic cups were pushed out on the sides.
(I had picture of my experiment but I was unable to get them to load onto my computer)
Water is smallest when it is at liquid form at room temperature
What happens when salt is added to the water?
When salt is added to the water it takes a lot longer for the water to completely freeze.
Does the amount of salt added affect the freezing?
Yes, the more salt in the water the longer it take for the water to freeze.
For the experiment I had 3 plastic cups with 1 cup of water in each cup. I let the water sit at room temperature for 1 hour. The water's temperature was 16C. I marked the cups at the water level.
Cup 1- Water
Cup 2- Water with 1 shake of salt
Cup 3- Water with 1 spoonful of salt
I placed all 3 cups into the freezer. The freezers temp was -15 C. I left the cups in the freezer for 7 hours. When I checked the cups the water was frozen in all the cups except for cup 3, that was the cup with a spoonful of salt. Cup 3 had a small layer of non-melted water sitting on top of the ice. I noticed that the plastic cups were pushed out on the sides.
(I had picture of my experiment but I was unable to get them to load onto my computer)
Activity 5 1-19-11 pH
Describe pH, present a table of pH ranges for some common things.


If something is pH 3 and something else is pH 4, which is more acidic and by how much?
Ph3 is more acidic than Ph4. Ph3 is 10 times more acidic than Ph4.
Ph3 is more acidic than Ph4. Ph3 is 10 times more acidic than Ph4.
Activity 4 1-19-11 Dissolving
Show an image or animation or description of what is happening when water dissolves NaCl.
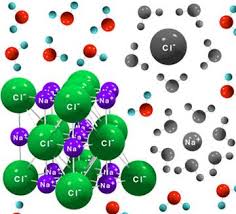
Why is the freezing point lowered when salt is added to water?
When salt is added to water it lowers the freezing point. The water molecules form crystals when freeze. The salt gets in the way of the water molecules, making it harder for the water molecules to become crystals again. This then means that salt water remain a liquid for a longer time as the temperature gets colder.
When salt is added to water it lowers the freezing point. The water molecules form crystals when freeze. The salt gets in the way of the water molecules, making it harder for the water molecules to become crystals again. This then means that salt water remain a liquid for a longer time as the temperature gets colder.
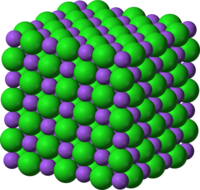
Activity 3 1-19-11 Crystal Structures
Show crystal structures fo NaCl (sodium chloride)and CaCO3 (calcium carbonate). what is the melting point for these two materials.
NaCl (Sodium chloride)

CaCO3 (Calcium carbonate)

Melting Points:
Sodium Chloride = 801 °C, 1074 K, 1474 °F
Calcium Carbonate = 825 °C, 1098 K, 1517 °F
NaCl (Sodium chloride)
CaCO3 (Calcium carbonate)
Melting Points:
Sodium Chloride = 801 °C, 1074 K, 1474 °F
Calcium Carbonate = 825 °C, 1098 K, 1517 °F
Activity2 1-19-11 Weather and Bernouli Principle
Describe some common weather occurences in relation to the gas laws and characteristics we have been exploring.
Humid air is much less dense than dry air.
Humid air is much less dense than dry air.
What is the Bernouli Principle, provide an example or application of this.

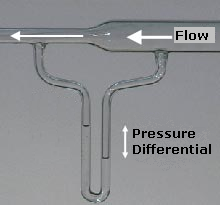
Bernoulli's principle states that for an inviscid flow, an increase in the speed of the fluid occurs simultaneously with a decrease in pressure or a decrease in the fluid's potential energy. Bernoulli's principle can be applied to various types of fluid flow, resulting in what is loosely denoted as Bernoulli's equation. In fact, there are different forms of the Bernoulli equation for different types of flow. The simple form of Bernoulli's principle is valid for incompressible flows (e.g. most liquid flows) and also for compressible flows (e.g. gases) moving at low Mach numbers. More advanced forms may in some cases be applied to compressible flows at higher Mach numbers.

Activity 1 1-19-11 Ideal Gas Law
What is the tire pressure at -20F compared to 90F, assuming the starting pressure was 32 psi?
F to Kelvin
90 F = 305.2 Kelvin
-20 F = 244.3 Kelvin
32 psi/ (244.3 K) =7817.6/ 305.2 K = 25.61 psi
Tuesday, January 18, 2011
Activity 2 1-18-11 Molecular Masses
List the molecular masses of carbon dioxide, oxygen, and helium. Explain how you get this from the Periodic Table.
Carbon dioxide-CO2 44
12+16+16=44g/mole
Oxygen-O2 32
16 + 16=32g/mole
Helium-He 4
4g/mole
Water-H2O 18
1+1+16= 18g/mole
Carbon dioxide-CO2 44
12+16+16=44g/mole
Oxygen-O2 32
16 + 16=32g/mole
Helium-He 4
4g/mole
Water-H2O 18
1+1+16= 18g/mole
Activity 1 1-18-11 Burning Candle Observations
What is burning? Show the chemical formula and an image of the structure.
The heat from the wick starts to melt and burn the wax around the wick.

Write the chemical reaction.
C25H52 + 38 O2 ------- 25CO2 + 26 H2O + energy (heat and light)
The chemical formula for candel wax C25 H52
standard combustion reaction. i.e. CxHy + (x + y/4) O2 ---> (y/2) H2O + (x) CO2
The chemical formula for candel wax C25 H52
The solid forms of paraffin, called paraffin wax, are from the heaviest molecules from C20H42 to C40H82.
Write the balanced chemical reaction.
Butane Water Carbon Dioxide
2C4H10 + 13O2 -------->10H2O + 8CO2
C25 H52 + 38O2 -------------> 26H2O +25CO2
Example from web:
Unbalanced: C25H52 + O2 → CO2 + H2O
Balanced: C25H52 + 38 O2 → 25 CO2 + 26 H2O
Balanced: C25H52 + 38 O2 → 25 CO2 + 26 H2O
Additional infomation I found on the web:
An interesting thing about a candle is that it is a very good example of the four states of matter. The four states of matter are Solid, Liquid, Gas, and Plasma. Wax, the fuel source of a candle goes through all four of these states of matter. Let's see what happens when we light a candle. We need a catalyst to start the whole thing going, so we touch a flame to the end of the wick. At first the wick itself starts to burn. The flame creeps down the sides of the wick and usually diminishes until it touches the wax. This is when the mechanism starts to work. The heat from the flame melts the wax surrounding the base of the wick directly beneath it. By the principle of capillary action the melted wax is drawn into the wick providing fuel for the flame which bursts to life. The candle continues to burn through an ongoing cycle. Wax in a solid state of matter is melted by the heat of the flame and converts it to a liquid state of matter. The liquid wax is drawn up to the tip of the wick inside the flame. At this stage the liquid wax is heated even more and it vaporizes changing into a gaseous state of matter. The gaseous wax enters the combustion area of the flame and is converted to energy. The energy conversion gives off heat, which melts more of the solid wax. The cycle repeats itself until the wick no longer functions due to consumption or lack of fuel for the flame
Sunday, January 16, 2011
Homework 1-12-11
Prepare to present the results of the Liquids/Surfaces experiment.
1. Contact solution on wood table.
When I observed the contact solution spread out fast at first, then settled into a circle. I used a nickle for a size comparason. The contact solution stayed in a circle on the wood table. The circle was slightly smaller than a nickel and not as thick.
2. Contact solution on wood table with oil.
For the second experiment I first covered the wood table with cooking spray to give the surface an oily feeling. When I dropped the contact solution onto the surface it splattered and did not make the perfect circle like the first experiment without the oil. The contact solution on the oily table had oval shape and was very thin. The contact solution was slightly smaller and much thinner than a nickel.
 3. Mouthwash on a paper plate.
3. Mouthwash on a paper plate.
The mouthwash soaked through the paper plate, with a small puddle in the middle leaving a ring around the puddle. The mouthwash made a perfect circle. The puddle in the middle of the plate was small and the ring around the puddle was about the size of the nickel but much thinner than the nickle. Most of the mouthwash soaked into the plate after only a few seconds.
4. Soapy water on a plastic cutting board.
The soapy water on the plastic cutting board was not a perfect circle. The water moved outward the longer it sat on the surface, the water was a very free moving liquid on the plastic surface. The water was hard to measure because it moved around so much. The water was bigger than a nickel and more like the size of a quarter. The shape of the water was odd and thin.
5. Pure vegetable oil on plastic surface.
The vegetable oil stayed in a perfect circle and had good form. The vegetable oil settled and stayed in one place. The vegetable oil is hard to see in the picture but it is the size of a nickel. The top of the oil was as thick as a nickel.
6. Sanatizer on Styrofoam Bowl
The sanitizer on the styrofoam bowl was a perfect circle. It didn't spread out as it hit the plate. It sits on the surface without moving even when the bowl was tilted. The sanitizer was much smaller than a nickle in size, and smaller than a penny. The sanitizer was not as thick as a penny or a nickel.
7. Wine on tupperware container (plastic)
The wine on the tupperware container was a perfect circle. It moved around when it was tilted. It was about the same size as a penny, and was about as thick as a penny
8. Wine on tupperware container with oil (cooking spray)
For the eighth experiment I put cooking spray on the plastic tupperware container to give it an oily feeling. When poured, the wine did not make a perfect circle like in the last experiment. The wine spread to wherever the cooking spray was. The wine on the oil surface was bigger than a penny and bigger than a nickel.
10. Sanitizer on a glass surface with oil
When the sanitizer was put into the container it formed circles, but then it began to spread out into the oil. The circles seemed to be consistent in size, and were a little bit smaller then the size of a penny.
Table of data collected.
Circle shape: Experiments 1, 3, 5, 6, 7, and 10
Non-circle shape: Experiments 2, 4, 8, and 9.
Size comparisons of the liquids.
Smaller than a penny: Experiments 6 and 10.
Same size as a penny: Experiments 7 and 9.
Bigger than a penny: Experiments 1 and 2.
Same size as a nickel: Experiments 3 and 5.
Bigger than a nickel: Experiments 4 and 8.
Conclusion:
After doing 10 different experiments I have detemined that different liquids produce different shapes and sizes when they are on different surfaces. I noticed that putting oil on the different surfaces directly effected the liquids size and shape when put on top of the oils. Certain liquids change width and height when on different surfaces. I thought it was interesting that each size category had 2 experiements in each of the categories. I also noticed that more of the experiments had circle shapes than non-circle shapes. If I were going to do another experiment like this agian I would try all the liquids on each of the surfaces so I could see the differences between the liquids.
Items to present:
1. pictures of observations
2. collected data
3. data in a graph/plot/table
4. conclusions based on data related to the molecular attractions and/or repulsions of the various chemicals or materials observed.1. Contact solution on wood table.
When I observed the contact solution spread out fast at first, then settled into a circle. I used a nickle for a size comparason. The contact solution stayed in a circle on the wood table. The circle was slightly smaller than a nickel and not as thick.
2. Contact solution on wood table with oil.
For the second experiment I first covered the wood table with cooking spray to give the surface an oily feeling. When I dropped the contact solution onto the surface it splattered and did not make the perfect circle like the first experiment without the oil. The contact solution on the oily table had oval shape and was very thin. The contact solution was slightly smaller and much thinner than a nickel.

The mouthwash soaked through the paper plate, with a small puddle in the middle leaving a ring around the puddle. The mouthwash made a perfect circle. The puddle in the middle of the plate was small and the ring around the puddle was about the size of the nickel but much thinner than the nickle. Most of the mouthwash soaked into the plate after only a few seconds.
4. Soapy water on a plastic cutting board.
The soapy water on the plastic cutting board was not a perfect circle. The water moved outward the longer it sat on the surface, the water was a very free moving liquid on the plastic surface. The water was hard to measure because it moved around so much. The water was bigger than a nickel and more like the size of a quarter. The shape of the water was odd and thin.
5. Pure vegetable oil on plastic surface.
The vegetable oil stayed in a perfect circle and had good form. The vegetable oil settled and stayed in one place. The vegetable oil is hard to see in the picture but it is the size of a nickel. The top of the oil was as thick as a nickel.
6. Sanatizer on Styrofoam Bowl
The sanitizer on the styrofoam bowl was a perfect circle. It didn't spread out as it hit the plate. It sits on the surface without moving even when the bowl was tilted. The sanitizer was much smaller than a nickle in size, and smaller than a penny. The sanitizer was not as thick as a penny or a nickel.
7. Wine on tupperware container (plastic)
The wine on the tupperware container was a perfect circle. It moved around when it was tilted. It was about the same size as a penny, and was about as thick as a penny
8. Wine on tupperware container with oil (cooking spray)
For the eighth experiment I put cooking spray on the plastic tupperware container to give it an oily feeling. When poured, the wine did not make a perfect circle like in the last experiment. The wine spread to wherever the cooking spray was. The wine on the oil surface was bigger than a penny and bigger than a nickel.
9. Soapy water on tupperware container with oil
What forms into various circles, which are not perfect. When I lightly shook the container it didn't cause the liquid to move. The various circles were about the same size of a penny.
When the sanitizer was put into the container it formed circles, but then it began to spread out into the oil. The circles seemed to be consistent in size, and were a little bit smaller then the size of a penny.
Table of data collected.
Circle shape: Experiments 1, 3, 5, 6, 7, and 10
Non-circle shape: Experiments 2, 4, 8, and 9.
Size comparisons of the liquids.
Smaller than a penny: Experiments 6 and 10.
Same size as a penny: Experiments 7 and 9.
Bigger than a penny: Experiments 1 and 2.
Same size as a nickel: Experiments 3 and 5.
Bigger than a nickel: Experiments 4 and 8.
Conclusion:
After doing 10 different experiments I have detemined that different liquids produce different shapes and sizes when they are on different surfaces. I noticed that putting oil on the different surfaces directly effected the liquids size and shape when put on top of the oils. Certain liquids change width and height when on different surfaces. I thought it was interesting that each size category had 2 experiements in each of the categories. I also noticed that more of the experiments had circle shapes than non-circle shapes. If I were going to do another experiment like this agian I would try all the liquids on each of the surfaces so I could see the differences between the liquids.
Activity 1 1-13-11 Surface Chemical MAke-up
What is the chemicals and their structures that make up cardboard?
What is the chemicals and their structures that make up waxes?
Like paper, cardboard is made from cellulose fiber from trees. Cellulose is a complex carbohydrate with chemical formula (C6H10O5)n.
What is the chemicals and their structures that make up styrofoam?
Styrofoam is made of polystyrene.
The chemical makeup of polystyrene is a long chain hydrocarbon with every other carbon connected to a phenyl group (the name given to the aromatic ring benzene, when bonded to complex carbon substituents). Polystyrene's chemical formula is (C8H8)n; it contains the chemical elements carbon and hydrogen. Because it is an aromatic hydrocarbon, it burns with an orange-yellow flame, giving off soot, as opposed to non-aromatic hydrocarbon polymers such as polyethylene, which burn with a light yellow flame (often with a blue tinge) and no soot. Complete oxidation of polystyrene produces only carbon dioxide and water vapor. Because of its chemical inertness, polystyrene is used to fabricate containers for chemicals, solvents, and foods.
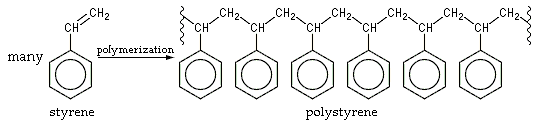
The chemical makeup of polystyrene is a long chain hydrocarbon with every other carbon connected to a phenyl group (the name given to the aromatic ring benzene, when bonded to complex carbon substituents). Polystyrene's chemical formula is (C8H8)n; it contains the chemical elements carbon and hydrogen. Because it is an aromatic hydrocarbon, it burns with an orange-yellow flame, giving off soot, as opposed to non-aromatic hydrocarbon polymers such as polyethylene, which burn with a light yellow flame (often with a blue tinge) and no soot. Complete oxidation of polystyrene produces only carbon dioxide and water vapor. Because of its chemical inertness, polystyrene is used to fabricate containers for chemicals, solvents, and foods.
What is the chemicals and their structures that make up waxes?
Wax refer to a class of chemical compounds that are plastic (malleable) near ambient temperatures. Characteristically, they melt above 45 °C (113 °F) to give a low viscosity liquid. Waxes are insoluble in water but soluble in petroleum based solvent. All waxes are organic compounds, both synthetic and naturally occurring.

Explain why water is attracted to cardboard and not styrofoam.
Water is attracted to cardboard because of the electronegativity.
Water is attracted to cardboard because of the electronegativity.
Activity 5 1-13-11 Pressure
How much pressure do you place on someone when you stand on them?
lbs = 200 in^2 = 12 200lbs/12 in^2 = 16.66 lbs/in^2
lbs = 200 in^2 = 12 200lbs/12 in^2 = 16.66 lbs/in^2
Thursday, January 13, 2011
Activity 3 1-13-11 What is Soap?
Show a chemical structure for soap.
It is difficult to wash an oil spot out of clothing with plain water, because oil is a hydrocarbon that does not dissolve in water. Oil and water actually repel one another, so that oil adheres even more strongly to clothing in the presence of water. The addition of soap or detergent to water changes the situation; soapy water can dissolve oil from clothing and rinse it away. What is special about the structure of soaps that makes them effective cleaning agents for oils and greases?
Most soaps are soluble sodium or potassium salts of carboxylic acids. The most common commercial soap is sodium stearate, Na[C17H35CO2]. It dissolves in water, forming the sodium and stearate ions. Even though most of the stearate ion is a hydrocarbon chain, it dissolves in water because of the carboxylate group. The carboxylate end is called hydrophilic (water-loving), and the hydrocarbon tail is called hydrophobic (water-fearing).

It is difficult to wash an oil spot out of clothing with plain water, because oil is a hydrocarbon that does not dissolve in water. Oil and water actually repel one another, so that oil adheres even more strongly to clothing in the presence of water. The addition of soap or detergent to water changes the situation; soapy water can dissolve oil from clothing and rinse it away. What is special about the structure of soaps that makes them effective cleaning agents for oils and greases?
Most soaps are soluble sodium or potassium salts of carboxylic acids. The most common commercial soap is sodium stearate, Na[C17H35CO2]. It dissolves in water, forming the sodium and stearate ions. Even though most of the stearate ion is a hydrocarbon chain, it dissolves in water because of the carboxylate group. The carboxylate end is called hydrophilic (water-loving), and the hydrocarbon tail is called hydrophobic (water-fearing).

What is unique about the polarity(charge characteristics) of soap?
It is the long hydrocarbon chains of the stearate anions that dissolve the oils and greases. If water containing dissolved soap is mixed with oil, the hydrocarbon chains strongly attract the oil, while the ionic ends keep the soap dissolved into water. The oil spot is broken up into small droplets and dispersed into the water. The "tails" of many soap anions are needed to remove each oil droplet.
While the sodium salt of stearate ions and the anions of other soaps are soluble in water, the calcium and magnesium salts are not. Hard water contains these metal cations, so the metal salts precipitate, reducing the oil-dissolving efficiency of the soap. "Bathtub ring" originates from the precipitation of soap by hard water. Thus, soaps do not clean well in hard water until most of the metal cations have been precipitated by reacting with the soap. In recent years, this problem has been solved by replacing soaps with detergents, which are generally compounds with long hydrophobic tails and the charged sulfate group such as sodium dodecyl sulfate, Na[CH3(CH2)11OSO3]. The calcium and magnesium salts of detergents generally remain soluble in water.
While the sodium salt of stearate ions and the anions of other soaps are soluble in water, the calcium and magnesium salts are not. Hard water contains these metal cations, so the metal salts precipitate, reducing the oil-dissolving efficiency of the soap. "Bathtub ring" originates from the precipitation of soap by hard water. Thus, soaps do not clean well in hard water until most of the metal cations have been precipitated by reacting with the soap. In recent years, this problem has been solved by replacing soaps with detergents, which are generally compounds with long hydrophobic tails and the charged sulfate group such as sodium dodecyl sulfate, Na[CH3(CH2)11OSO3]. The calcium and magnesium salts of detergents generally remain soluble in water.
How does soap work?
Nearly all compounds fall into one of two categories: hydrophilic ('water-loving') and hydrophobic ('water-hating'). Water and anything that will mix with water are hydrophilic. Oil and anything that will mix with oil are hydrophobic. When water and oil are mixed they separate. Hydrophilic and hydrophobic compounds just don't mix.
The cleansing action of soap is determined by its polar and non-polar structures in conjunction with an application of solubility principles. The long hydrocarbon chain is non-polar and hydrophobic (repelled by water). The "salt" end of the soap molecule is ionic and hydrophilic (water soluble).
When grease or oil (non-polar hydrocarbons) are mixed with a soap- water solution, the soap molecules work as a bridge between polar water molecules and non-polar oil molecules. Since soap molecules have both properties of non-polar and polar molecules the soap can act as an emulsifier. An emulsifier is capable of dispersing one liquid into another immiscible liquid. This means that while oil (which attracts dirt) doesn't naturally mix with water, soap can suspend oil/dirt in such a way that it can be removed. The soap will form micelles (see below) and trap the fats within the micelle. Since the micelle is soluble in water, it can easily be washed away
Nearly all compounds fall into one of two categories: hydrophilic ('water-loving') and hydrophobic ('water-hating'). Water and anything that will mix with water are hydrophilic. Oil and anything that will mix with oil are hydrophobic. When water and oil are mixed they separate. Hydrophilic and hydrophobic compounds just don't mix.
The cleansing action of soap is determined by its polar and non-polar structures in conjunction with an application of solubility principles. The long hydrocarbon chain is non-polar and hydrophobic (repelled by water). The "salt" end of the soap molecule is ionic and hydrophilic (water soluble).
When grease or oil (non-polar hydrocarbons) are mixed with a soap- water solution, the soap molecules work as a bridge between polar water molecules and non-polar oil molecules. Since soap molecules have both properties of non-polar and polar molecules the soap can act as an emulsifier. An emulsifier is capable of dispersing one liquid into another immiscible liquid. This means that while oil (which attracts dirt) doesn't naturally mix with water, soap can suspend oil/dirt in such a way that it can be removed. The soap will form micelles (see below) and trap the fats within the micelle. Since the micelle is soluble in water, it can easily be washed away
Activity 2 1-13-11 Vitamin Solubllity
Show the chemical structure of vitamin C and Vitamin E and describe which one is water soluble and which is fat soluble.
Vitamin C

Vitamin E

Vitamin C is water soluble.
The two types of vitamins are classified by the materials in which they will dissolve. Fat-soluble vitamins -- vitamins A, D, E and K -- dissolve in fat before they are absorbed in the blood stream to carry out their functions. Excesses of these vitamins are stored in the liver. Because they are stored, they are not needed every day in the diet.
By contrast, water-soluble vitamins dissolve in water and are not stored; they are eliminated in urine. We need a continuous supply of them in our diets. The water-soluble vitamins are the B-complex group and vitamin C.
Vitamin E is fat soluble.
Vitamin E is found naturally in some foods, added to others, and available as a dietary supplement. "Vitamin E" is the collective name for a group of fat-soluble compounds with distinctive antioxidant activities.
Vitamin C

Vitamin E

Vitamin C is water soluble.
The two types of vitamins are classified by the materials in which they will dissolve. Fat-soluble vitamins -- vitamins A, D, E and K -- dissolve in fat before they are absorbed in the blood stream to carry out their functions. Excesses of these vitamins are stored in the liver. Because they are stored, they are not needed every day in the diet.
By contrast, water-soluble vitamins dissolve in water and are not stored; they are eliminated in urine. We need a continuous supply of them in our diets. The water-soluble vitamins are the B-complex group and vitamin C.
Vitamin E is fat soluble.
Vitamin E is found naturally in some foods, added to others, and available as a dietary supplement. "Vitamin E" is the collective name for a group of fat-soluble compounds with distinctive antioxidant activities.
Homework 1-11-11
1. Contact Solution on Wood Table
2. Contact Solution on Wood Table with Oil on the table
3. Mouthwash on Paper Plate 4. Soapy Water on Plastic
5. Pure Vegatable oil on Plastic Surface
6. Sanitizer on Styrofoam
7. Wine on Tupperware Container (plastic)
8. Wine on Tupperware Container (plastic) with Oil
9. Soapy Water on Tupperware Container with Oil
10. Sanatizer on Glass Surface with Oil
2. Contact Solution on Wood Table with Oil on the table
3. Mouthwash on Paper Plate 4. Soapy Water on Plastic
5. Pure Vegatable oil on Plastic Surface
6. Sanitizer on Styrofoam
7. Wine on Tupperware Container (plastic)
8. Wine on Tupperware Container (plastic) with Oil
9. Soapy Water on Tupperware Container with Oil
10. Sanatizer on Glass Surface with Oil
Subscribe to:
Posts (Atom)