What is the chemicals and their structures that make up styrofoam?
Styrofoam is made of polystyrene.
The chemical makeup of polystyrene is a long chain hydrocarbon with every other carbon connected to a phenyl group (the name given to the aromatic ring benzene, when bonded to complex carbon substituents). Polystyrene's chemical formula is (C8H8)n; it contains the chemical elements carbon and hydrogen. Because it is an aromatic hydrocarbon, it burns with an orange-yellow flame, giving off soot, as opposed to non-aromatic hydrocarbon polymers such as polyethylene, which burn with a light yellow flame (often with a blue tinge) and no soot. Complete oxidation of polystyrene produces only carbon dioxide and water vapor. Because of its chemical inertness, polystyrene is used to fabricate containers for chemicals, solvents, and foods.
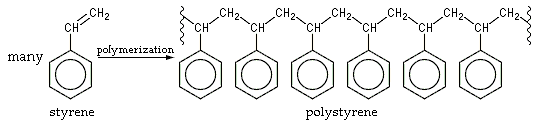
The chemical makeup of polystyrene is a long chain hydrocarbon with every other carbon connected to a phenyl group (the name given to the aromatic ring benzene, when bonded to complex carbon substituents). Polystyrene's chemical formula is (C8H8)n; it contains the chemical elements carbon and hydrogen. Because it is an aromatic hydrocarbon, it burns with an orange-yellow flame, giving off soot, as opposed to non-aromatic hydrocarbon polymers such as polyethylene, which burn with a light yellow flame (often with a blue tinge) and no soot. Complete oxidation of polystyrene produces only carbon dioxide and water vapor. Because of its chemical inertness, polystyrene is used to fabricate containers for chemicals, solvents, and foods.
What is the chemicals and their structures that make up waxes?
Wax refer to a class of chemical compounds that are plastic (malleable) near ambient temperatures. Characteristically, they melt above 45 °C (113 °F) to give a low viscosity liquid. Waxes are insoluble in water but soluble in petroleum based solvent. All waxes are organic compounds, both synthetic and naturally occurring.

Explain why water is attracted to cardboard and not styrofoam.
Water is attracted to cardboard because of the electronegativity.
Water is attracted to cardboard because of the electronegativity.
No comments:
Post a Comment